At Alpha Prolipsis, we are dedicated to providing you with easy access to the best in healthcare. Our doctors and medical team prioritise patient care and are well supported by warm and caring clinic staff. Our clinicians genuinely care about you and your health concerns. Alpha Prolipsis are all highly qualified professionals and are constantly engaging in further education and development. The founding shareholders were passionate about differentiating health care services through innovative solutions to improve the quality of patient care, improve health services within the community and to address the ever increasing challenges around Federal/State Health funding. Medicrew emerged as an innovative organisation that has successfully utilised and promoted the capabilities of leading medical clinicians.
Dafni
Phone
210 9756566
Psichikon
Phone
210 6980565
Glyfada
Phone
210 9610982
Gyzi
Phone
210 6444430
Pallini
Phone
210 6034681
Chaidarion
Phone
6977430971
ALPHA PROLIPSIS
Medical Laboratories - Polyclinics
ALPHA PROLIPSIS' medical laboratories network is contracted with EOPYY.




Health Offers
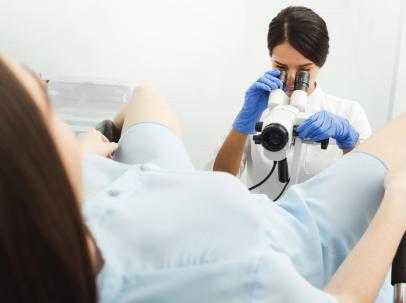
GYNECOLOGICAL EXAMINATION
The gynecological examination aims to determine the general state of a female's genital system health through clinical and targeted cytological and radiological examinations.
SPERM TEST (SPERM DIAGRAM)
The sperm chart identifies problems that are responsible for male infertility. The sperm schedule greatly influences the treating fertility doctor's decisions that you will need to follow.
URINE CYTOLOGY EXAMINATION
Urine cytology should be performed when cancer of the bladder, renal pelvis, ureters or urethra is suspected. It is also used to monitor patients who have been treated for transitional epithelial
Your reports will be available within 24 hours of receiving the sample
They said about us